Embryo development
During in vitro fertilization (IVF), sperm and eggs are either mixed together or a sperm is injected into an egg to form an embryo (intracytoplasmic sperm injection). After several days of development, the embryos are placed into the woman’s uterus. This is referred to as implantation.
Traditionally, the embryo is allowed to grow for two to three days prior to transfer. One of the problems with this approach is that there is very little to see that indicates which embryo has the best chance of implanting.
An alternative to day 3 transfers is to culture embryos for five or six days (the blastocyst stage) prior to transfer. This does two things:
- It allows the embryo to progress and grow to a more advanced stage so that one can make observations about the quality of two important types of cells in the embryo.
- The embryo grows to a stage where it must overcome a major metabolic hurdle – the development past the 8 to 12 cell stage.
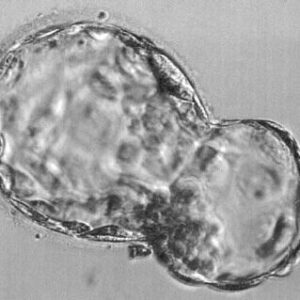
The two important cell types that are important for the development of a fetus are the inner cell mass and the trophectoderm cells. The blastocyst appears as a hollow ball with a clump of cells at one of its poles. This hollow ball is made of trophectoderm cells that later form parts of the placenta. These cells are necessary for implantation.
The clump of cells at one pole are called the inner cell mass. These are the cells that form the actual fetus. If an embryo contains only inner cell mass cells, the blastocyst cannot implant. If an embryo has only trophectoderm cells, the embryo can implant but a fetus is not formed and the result will be an early miscarriage.
Reasons for higher success rates
When we wait until day 5 or 6, it is much easier to see these two types of cells, and we can select those embryos that have the best trophectoderm and inner cell masses. Probably a more important reason to perform transfers at the blastocyst stage is that we have a better idea of the quality of the embryo just by the fact that it survived to day 5 or 6.
On average, only 30 to 50 percent of embryos make it to the blastocyst stage. The failure of some embryos to not make it to the blastocyst stage is most likely due to a defect in the embryo. If, for example, we have 10 embryos on day 3 and we select two to transfer on day 3, we may not select the right embryos.
The probability that we select the three or five that would have gone to day 5 or 6 is not very high. In this instance we will be transferring some day 3 embryos that really have no chance of making it to day 5 or 6. If instead we wait a few more days when we know which embryos are competent to make a blastocyst, we will increase our odds of the embryos implanting.
Most studies indicate the chance for a day 3 embryo to implant is about 20 percent. This is like having a six-sided dice and having to roll a one or a two for implantation to occur. It is much better to pick a blastocyst, where implantation rates are in the 50 percent range – like flipping a coin and having to decide between heads or tails.
Elective single embryo transfer (eSET)
The only way a day 3 embryo can approach the success of blastocysts is to transfer more embryos, which will increase the patient’s risk for having high-order multiples (triplets or above). Though some patients going through infertility treatment might want twins or higher multiple pregnancies, these are higher risk than single pregnancies.
Multiple pregnancies can cause serious complications and health risks to both mother and child. These risks can be reduced while maintaining good pregnancy rates if embryos are transferred with a higher potential for implantation (blastocysts) and if fewer embryos are transferred.
As we continue to improve our pregnancy rates at Arizona Reproductive Medicine Specialists, we remain steadfast in promoting single embryo transfers (eSET) to reduce multiple pregnancies.
The only down side to doing a blastocyst transfer is that there may be some risk that no embryos make it over the hurdle and survive to day 5. But, most likely, if none made it to blastocyst in the incubator, they would not do so inside of the woman, and the end result would be the same.
Frozen embryo transfer (FET)
There is also one other very important reason to let embryos develop to the blastocyst stage: Blastocysts freeze better than day 2 or 3 embryos. By freezing at the blastocyst stage, pregnancy can occur more quickly without the many futile frozen embryo transfers from day 3 embryos.
Many of the day 3 embryos when thawed will either not survive or are incapable of growing to day 5 or 6, either inside the uterus or outside the uterus. This not only saves time but also the patients’ money.
Programs that can freeze blastocysts successfully not only decrease the overall costs for their patients and increase their success, but they decrease the risks to a woman of getting ill or even dying. If an IVF program has a good freezing program, they can then depend on it for pregnancies.
This means they can transfer fewer embryos in the fresh cycle (reducing the risk of multiples), as well as not transferring embryos in a fresh cycle where either the patient’s uterus is not optimal (poor lining) or there is a chance of endangering the patient’s life. This can be the case, for example, when the patient is at risk for severe ovarian hyperstimulation syndrome. If a program cannot depend on frozen embryos, physicians may elect to take chances and transfer more embryos or transfer embryos into a woman who could die from being pregnant while suffering from severe ovarian hyperstimulation syndrome.

Frozen Embryo Transfer
Preimplantation genetic diagnosis (PGD)
Blastocyst transfer is also important for patients who are having preimplantation genetic diagnosis (PGD). This is when a couple has a genetic disease or are carriers for a genetic disease and they wish to reduce the chances of their children becoming affected with that disease.
In this case, embryos are cultured to day 3. Then a cell is removed from the embryo and is analyzed to determine if the embryo has the disease of interest. This analysis can take days, so the embryos are cultured to day 5 or 6 and transferred at that time after the analysis is completed. With new developments in molecular biology, IVF clinics are now even turning to doing these diagnoses on blastocysts, where there are more cells to choose from and there is a lower likelihood of harming the embryo.